Background
Chronic graft-versus-host disease (cGVHD) is the leading cause of late morbidity and mortality as well as impaired quality of life after allogeneic hematopoietic stem cell transplantation (allo-SCT). Established first-line therapy for cGVHD still comprises corticosteroids and calcineurin inhibitors. However, about half of the patients with cGVHD are refractory to therapy with corticosteroids and the response rates ranges from 30% to 60%. No consensus has been reached regarding the optimal salvage treatment for steroid-refractory (SR)-cGVHD. Studies have reported that ruxolitinib (RUX, Jakafi, Novartis, Basel, Switzerland), a selective Janus kinase 1/2 (JAK1/JAK2) inhibitor, prevents GVHD but preserves the beneficial graft versus leukemia (GvL) effect. Here, we present study investigating the clinical efficacy of ruxolitinib and its safety profile in patients having SR-cGVHD after allo-SCT during the treatment course.
Methods
This was a retrospective study and all data were collected from the clinical history of a single center - the First Affiliated Hospital of Zhejiang University School of Medicine. Patients treated with ruxolitinib for SR-cGVHD after allo-SCT between August 2017 and December 2019 were involved. A two-tailed P value of 0.05 was considered statistically significant. Univariate comparisons of parameters were performed using the χ2 test, Fisher exact test, and Studentttest,as appropriate. A multivariate model was used to calculate the odds ratio (OR) with a 95% confidence interval (CI). Overall survival (OS) was estimated and plotted using the Kaplan-Meier method.
Results
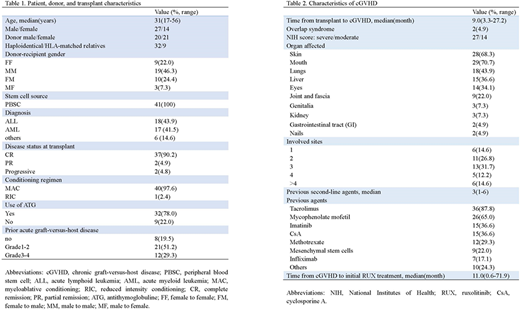
A total of 41 patients with SR-cGVHD were eligible. The demographic and baseline characteristics of patients were summarized in Table 1, and GVHD information was presented in Table 2. After a median duration of 8 months (range, 1.1-24.9) of ruxolitinib treatment, the overall response rate (ORR) was 73.2% (30/41), including 15 patients (36.6%) with complete response (CR) and 15 patients (36.6%) with partial response (PR). Lung cGVHD (OR, 0.138; 95% CI, 0.024-0.803; P=0.028) and matched related donors (OR, 0.129; 95% CI, 0.020-0.835; P = 0.032) had independent adverse effects on treatment response. Major adverse events associated with ruxolitinib were cytopenias and infectious complications. Cytopenias were reported during ruxolitinib treatment in six patients (5/41, 12.2%), of which three were (7.3%) of grades 3-4. A total of 23 patients developed any grade infections: 19 Epstein-Barr virus (EBV) DNAemia, five cytomegalovirus (CMV) DNAemia, two carbapenem-resistant Klebsiella pneumoniae sepsis, and one hepatitis B (HBV) reaction. The 6-month and 12-month OS rates for all treated patients were 87.8% (95% CI, 77.3-98.3) and 61.0% (95% CI, 45.4-76.6), respectively. The median follow-up for this cohort was 14.9 months. Prolonged survival was observed in patients with a male donor, CR before transplantation, baseline moderate cGVHD, and skin cGVHD.
Conclusion
This cohort showed the efficacy of ruxolitinib in SR-cGVHD treatment with the ORR of 73.2% and CR rate of 36.6%. Despite the limited sample size and retrospective nature, the results of this study indicated that patients with no lung involvement and haploidentical relatives as donors were more likely to benefit from ruxolitinib. Regarding the safety profile, the present study showed that the infection event was the most severe adverse effect related to ruxolitinib, highlighting the significance of infection prophylaxis.
No relevant conflicts of interest to declare.
ruxolitinib (RUX, Jakafi, Novartis, Basel, Switzerland)
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal